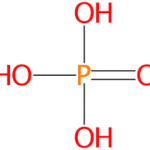
Phosphoric acid is also known by orthophosphoric acid in the market. It is widely used in manufacture of phosphate salts and in production of fertilisers. It is used as dental cement and also widely used in textile and sugar industries. It is available in the market as colourless, odourless, non-volatile liquid. Pure form of phosphoric acid is crystalline in room temperature and pressure and in a less concentrated form it is available as colourless liquid.
There are 3 salts of phosphoric acid, depending on the number of hydrogen atoms: sodium dihydrogen phosphate (NaH2PO4), used for control of hydrogen ion concentration of solutions; disodium hydrogen phosphate (Na2HPO4), used in water treatment as a precipitant for highly charged metal cations; trisodium phosphate (Na3PO4), used in soaps and detergents; calcium dihydrogen phosphate or calcium superphosphate (Ca[H2PO4]2), a major fertilizer ingredient; calcium monohydrogen phosphate (CaHPO4), used as a conditioning agent for salts and sugars.